Introduction
Cosmetics are commonly purchased products, considered nowadays to be necessities, and the cosmetics industry is one of the rapidly growing industries. It is estimated by Statista (2019) to be worth USD 545 billion in 2027, compared to USD 345 billion in 2018. Currently, one of the main trends shaping the industry is natural cosmetics. According to the Mintel agency (2020), between 2020 and 2030 consumers will be expecting the so-called “clean cosmetics”, which will pertain not only to their composition, but also to the environmental and social impacts throughout the product’s life cycle. This is the result of a higher social awareness – more and more consumers realize that making purchases is not only an economic act, but it also causes certain impact on other participants in the economy (Trivedi, 2019). Additionally, according to the Organic Trade Association (2021), the current strengthening of the natural cosmetics trend has been influenced by, among other things, the pandemic and the increased interest in environmentally- and health-friendly solutions.
However, with the increase in demand for natural products, unfair practices of manufacturers can be increasingly observed. There is a tendency to misuse the term “natural cosmetics” to refer to products with a low content of ingredients of natural origin (Żyngiel and Platta, 2014; Pawlik et al., 2017), and the word “natural” is often used in a persuasive way in advertisements (Gajewska, 2020). Moreover, cosmetic manufacturers often use the terms “natural”, “organic” and “vegan” interchangeably, resulting in confusion among consumers (Hsu, Chang and Yansritakul, 2017).
The paper consists of three theoretical chapters describing: types of product claims, legal requirements, self-regulations and good practices in this area, and two research chapters characterizing the research material, method and findings. Conclusions are included at the end.
Product claims
Among many tools influencing the consumer at a point of sale, packaging is the most important and constitutes a key element of the marketing communication system, treated as a system of signs. It is a channel of message and a carrier of coded information about the product. It plays an important role in the consumer’s decision-making process. This also applies to natural cosmetics, on the packages of which – apart from obligatory marks – manufacturers like to place various claims or logos suggesting e.g., functions and advantages of the products. These claims often encourage consumers to purchase a given cosmetic product, and many of them make purchase decisions based on them (Chandon, 2020; Naturativ, 2018). Claims are therefore an excellent marketing tool and can increase a company’s competitiveness, which is why a trend can be observed to place more and more claims on the packaging of new products (Cousté, Partal and Martínez-Ros, 2012).
As reported by Fowler, Reisenwitz, and Carlson (2015), the typology of product claims made on cosmetics packaging distinguishes:
– scientific claims, which present the results of clinical studies conducted and also provide data on the product formula, e.g., “dermatologically tested”, “contains 95% of aloe vera”;
– efficacy claims, which refer to subjective efficacy, mainly sensory, without any supporting evidence, e.g., “leaves it smooth and supple”;
– endorsement claims, which refer to the recommendation and acceptance of the product offered by e.g., celebrities, doctors, cosmetologists, certification organizations, e.g., “product approved by dermatologists”, “COSMOS Organic”;
– environmental claims, which focus on the environmentally-friendly features of a product, e.g., „95% ingredients of natural origin”, „vegan”;
– superiority claims, which focus on highlighting that the product is better than others, e.g., “product contains no colorants that may cause allergies”, “free of SLS”;
– emotional claims, which include exaggerated, metaphorical statements that are not understood literally by most consumers and therefore do not need to be justified, e.g., “for positive people”, “I am a specialist in my field”.
Product claims are defined in legal documents. Regulation (EC) No 1223/2009, which is the basic act and to which all cosmetic products, including natural cosmetics, are subject, states that product claims take the form of text, names, marks and images appearing on the label of the product and are used in it’s a
dvertising. Commission Regulation (EU) No 655/2013, on the other hand, specifies that product claims are signs presented in both textual and iconic form that serve to inform the consumer about the properties and characteristics of products, and are the primary means of distinguishing them. They relate to the function, composition and performance of a product, and the information they convey must be useful, understandable and reliable, and enable consumers to make informed decisions and choose products that meet their needs and expectations. They have an informative role at the stages of labelling, marketing and advertising cosmetics. Furthermore, the act lays down detailed criteria for substantiating claims used with reference to cosmetics.
Cosmetic product claims – legal requirements
The aforementioned Commission Regulation (EU) No 655/2013 is the basic act defining the requirements for making claims on cosmetic products. It is in line with other horizontal legislations on ethical communication, including cosmetic products and marketing claims on their packaging, i.e., the Unfair Commercial Practices Directive (UCPD), which aims to protect consumers and their economic interests, and the Misleading and Comparative Advertising Directive (MCAD), which aims to protect entrepreneurs against unfair commercial practices in the form of advertising.
In compliance with the aforementioned regulation, claims have to comply with the requirements which are as follows (Commission Regulation (EU) No 655/2013; Sub-Working Group on Claims, 2017):
- legal compliance – claims made by the manufacturer must comply with applicable legal regulations. It is not permissible to include information that a cosmetic product has been authorized or approved by an EU body, as all products available on the EU market must obligatorily meet the requirements and do not require approval by appropriate authorities, e.g., the claim “the product has been submitted to the Cosmetic Product Notification Portal” is incorrect as every product has to be submitted to the above-mentioned portal. The claims presented have to be adapted to the perception of the average consumer – taking into account social, cultural and linguistic factors. In addition, a product benefit cannot be claimed if it only refers to compliance with the minimum requirements contained in legal documents, e.g., “Lilial-free” is not allowed as the EU legislation prohibits the marketing of cosmetic products containing this substance;
- truthfulness – claims may not be made on the basis of false statements, e.g., if the manufacturer states “12-hour moisturizing”, they should provide evidence confirming the moisturizing time. On the other hand, claims concerning the content of a given ingredient must be confirmed in the composition of the product, e.g., if the manufacturer claims that a cosmetic contains honey, the product must contain honey and not its aroma. Claims relating to the properties of a specific ingredient should not indicate that the ready cosmetic product has the same properties if it does not, e.g., the statement “contains brightening bakuchiol” is not allowed if the final product does not have brightening properties. In addition, claims must not indicate that the opinions expressed are objective statements unless the opinions are supported by relevant evidence;
- evidential support – claims must be supported by adequate and verifiable evidence. If research results are used to substantiate the claims, the research should be of a state-of-the-art character and be relevant to the product and the claimed benefits, consistent with appropriately designed and conducted research methods, and with ethical principles. The scope of evidence should be consistent with the type of claim. A wide range of evidence or corroboration is particularly important for claims where the lack of efficacy of the product may compromise safety, e.g., a claim about sun protection factor should be supported by the test results of the ready product in terms of its efficacy against UVB radiation. Claims which the average consumer does not take literally and which are clearly abstract, do not need to be substantiated, e.g., “the scent of the cream will take you to a freshly mown meadow”. Conversely, claims that transfer the properties of an ingredient to that of the ready product must be supported by sufficient and appropriate evidence, e.g., demonstrating the presence of the ingredient in an effective concentration;
- honesty – claims should not emphasize the properties of the product if they cannot be supported by available evidence. In addition, the claims used must not emphasize the unique properties of a product if similar products have the same properties, e.g., it must not be emphasized that perfumes do not contain any preservatives as most perfumes contain a large amount of alcohol, which makes the use of preservatives unnecessary. If a product should be used with other cosmetics for it to be effective, this needs to be specified;
- fairness – claims must be neutral and must not slander competitors or lawful ingredients, e.g., “suitable for allergy sufferers as it contains no preservatives” is inconsistent as it assumes that all preservatives are allergenic. Moreover, claims must not lead to confusion between a cosmetic and its competing product;
- informed decision-making – claims should state precisely what they refer to, be comprehensible to the average consumer and enable him/her to make an informed decision. The manufacturer should take into account the ability of potential users to understand the message as well as cultural, linguistic and social factors among consumers in a given market, e.g., it is not justified to use technical language if the product is not intended for professionals.
These criteria are the most important requirements for product claims on cosmetic packaging. However, cosmetic product communication is subject not only to legislation but also to self-regulation and good practices, all of which create what is known as a co-regulatory system. All these elements constitute a legal environment and together form a system that ensures a high level of consumer protection against misleading claims while protecting entrepreneurs against unfair market practices and unfair competition.
Self-regulations and good practices for cosmetic product claims
Since July 1, 2019, expanded cosmetic labeling guidelines developed by SubWorkingGroup on Claims have been in effect. This document was developed upon request of the EU Member States, which indicated that there was a need to clarify the requirements for certain claims, as they might be misleading. However, the document is not of a legal nature, as it is only a set of good practices and details the requirements for claims:
- “does not contain” – which should not be included if: it refers to ingredients commonly prohibited in cosmetic products; the product contains a given ingredient belonging to the group of ingredients which the manufacturer declares the absence of; the ingredient may be released from the product; the absence of the ingredient cannot be substantiated by evidence; the ingredient is usually not used in a given type of cosmetic product; the absence of an ingredient guarantees product properties that cannot be guaranteed; a given product contains multifunctional ingredients that may perform a function that, according to the claim, the product does not perform; the ingredient is presented negatively, in particular in terms of its safety. “Does not contain” claims are allowed when they can help a specific group of consumers make an informed decision, e.g., “free from animal ingredients” is useful for vegans;
- “hypoallergenic” – this claim may be placed when the cosmetic has been created to reduce its allergenic properties. It must not imply that the product guarantees the complete absence of the risk of an allergic reaction. If there is a risk that the consumer will not understand the meaning of this statement, explanatory information should be provided. The claim should be supported by scientific and reliable evidence which should be constantly updated.
Similar detailed criteria have been developed on behalf of the European Commission for the use of the “natural” and “organic” claims. The documents containing these guidelines are two standards developed by the International Organization for Standardization – ISO 16128-1: 2016, which contains the definitions of the natural ingredient, the ingredient of natural origin and the organic ingredient, and ISO 16128-2: 2016, which provides a methodology for calculating the indices of natural and organic origin as well as naturalness or organicity of a cosmetic product, and with which the aforementioned claims can be substantively supported. They are of international character and are applied on a voluntary basis, do not require certification and the related financial outlays.
Self-regulations as well as standards developed by various national entities, which supplement the binding regulations, are also an important element of the regulation of product claims. In addition, independent certification bodies have developed their own guidelines for natural cosmetics and certificates, which also count as product claims. They can provide consumers with a guarantee of the naturalness of the product and protect them against the increasingly common phenomenon of greenwashing. Their use by producers is voluntary and involves the necessity to incur financial expenses for the certification process. There are over a dozen such certification bodies in Europe, whose standards are consistent with European law, e.g., ECOCERT, Cosmebio, BDIH, NaTrue.
The above common requirements and guidelines developed for all cosmetic products notwithstanding, unfair practices by manufacturers regarding marketing claims are still observed.
Research material and method
The aim of the research was to analyze product claims in terms of their frequency of occurrence, to assess their compliance with legal requirements and the declared composition of cosmetics, which was analyzed according to International Nomenclature of Cosmetic Ingredients, and to identify abuses.
Unit packages of 51 shower gels offered in stores in Krakow were selected for the assessment, which by claims on their labels suggested that they are natural; they had e.g., the following phrases: “natural cosmetic”, “X% natural ingredients”, and were also displayed on store shelves as such products. The research material consisted of cosmetics of global brands, as well as of small manufactories.
The research material was selected due to market trends. As indicated by Nielsen (2020), products in the personal care category account for the largest share of value sales in the market and the value of this category is primarily influenced by the sale of soaps, intimate hygiene gels and shower gels. Moreover, according to PMR, during the pandemic, a trend of the so-called home-centricity, that is a decrease in interest in color cosmetics, and an increase in the sale of home spa and skincare cosmetics (Wiadomości Kosmetyczne, 2020). Due to the fact that intimate hygiene gels are intended mainly for women, universal shower gels were selected for the assessment.
All textual and textual-graphic claims placed on unit packages of the aforementioned products were assessed, presented in Polish and foreign languages, as manufacturers are not obliged to place marketing claims in the language of the country where the cosmetics were launched on the market. The claims that had a similar message, but were formulated differently, were treated as two claims. The analysis was based on photographic documentation including labels of cosmetics.
The methodology developed by Fowler, Reisenwitz and Carlson (2015), and taking into account two typologies of claims, was used to examine the relationship between the type of claims on the packaging of natural cosmetics and the presence of misleading content among them. The first, described earlier, divides claims by their subject matter, and distinguishes 6 groups of claims: scientific, efficacy, environmental, emotional, endorsement, and superiority. The other typology developed by Carlson et al. (1993) deals with the division of claims due to their potential of misleading the consumer. It distinguishes 4 categories of claims:
- vague claims, containing general statements that make it impossible to unequivocally assess their correctness, e.g. “I am eco”;
- omission claims, which omit information enabling the assessment of its correctness, e.g., “dermatologically tested” without indicating, for example, the sample or the research center;
- false claims, which have been invented and do not correspond to reality, e.g., “contains mango extract” when this extract is not part of the composition of the product;
- mixed claims, which cannot be clearly assigned to any of the above categories as they contain various misleading elements.
For the purposes of the research, fifth category was added – acceptable claims.
Research findings
Types and frequency of claims
As a result of the analysis, 393 product statements were identified. Their presence was found on the packaging of all tested cosmetics, and mainly related to the intended use of the cosmetic, the description of active ingredients, the content of ingredients of natural origin, the absence of certain ingredients, intended use for vegans, and the expected results. Most of the claims were short and concise, containing a few words. Slightly more elaborate were descriptions of the effects of the ingredients contained in the product. There were also graphic claims on packages, mostly certificates granted by independent bodies. The percentage share of the occurrence of different types of claims is shown in Figure 1.
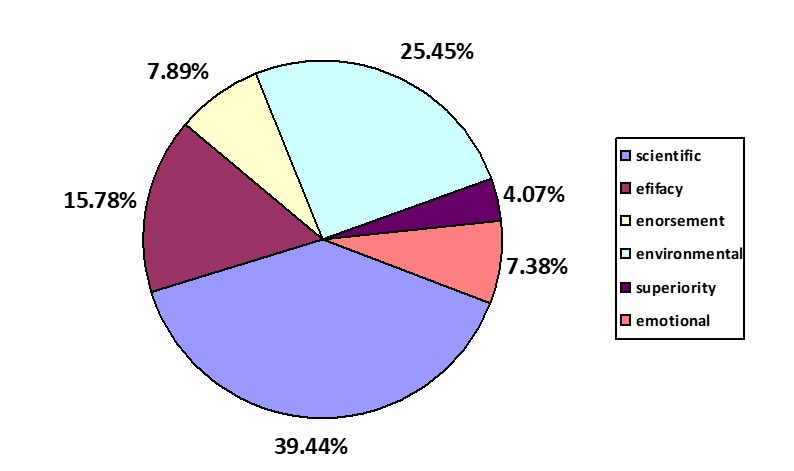
Fig 1: Percentage share of claims
Most of the claims were of a scientific nature (n=155). Among them, claims were distinguished that related to:
- description of the action of individual ingredients (n=47), e.g., “mint extract wonderfully tones the skin”,
- expected effects and general action of the product (n=42), e.g., “moisturizing gel”,
- formulation (n=35), i.e., the content of active ingredients, e.g., “recipe with an organic fig”,
- conducted dermatological tests, which should prove that the products have been tested with the participation of consumers under the supervision of a dermatologist, and that the companies have reports of the tests carried out (n=14),
- the intended use of the product (n=7), e.g., “for the most demanding sensitive and vascular skin”,
- the pH of the product (n=6),
- an indication that the product is suitable for the microbiome (n=2),
- the hypoallergenic properties (n=2).
The second most numerous group was environmental claims (n=100), which included claims about:
- the percentage of natural ingredients or of ingredients of natural origin (n=50),
- the product’s sustainability for vegans and/or vegetarians (without third-party certification) (n=24),
- the naturalness of the selected ingredient (n=13), e.g., “100% organic orange water essence”,
- the biodegradability of the product or any of its ingredients (n=9),
- no microplastics in the product (n=3),
- the product was not tested on animals (n=1).
Efficacy claims were the third group (n=62). They concerned:
- dermal sensations (n=39), e.g., “the gel gives a feeling of freshness”,
- skin scent (n=19), e.g., “leaves skin with the smell of mint”,
- the need to use a different product for a better sensory experience (n=4), e.g., “for a more thorough cleansing of the skin, use the Tahitian Mornings peeling”.
The fourth most numerous group comprised endorsement claims (n=31), among which the following can be distinguished:
- certificates confirming: the naturalness (n=15), organicity (n=2) and veganism (n=13) of the product, e.g., COSMOS Natural, The Vegan Society Trademark,
- approval by physicians, with an indication of the specific research center that issued the opinion on the product (n=1).
Another group comprised emotional claims (n=29) which were about:
- experiences (n=20), e.g., “a real feast for the senses”,
- product characteristics (n=5), e.g. “Your natural ally in the fight for the vitality skin”,
- purpose (n=4), e.g., “balm for the ever busy”.
The least numerous group comprised superiority claims, which informed about the lack of given groups of ingredients (n=16), e.g. “free from: SLS”.
Abuses
Analyzing the above-mentioned claims, 186 abuses were found. Most abuses occurred among the scientific claims (n=105). Many irregularities were also observed in the environmental claims (n=63). The lowest number of abuses was found among emotional claims (n=1). The frequency of irregularities is presented in Table 1.
Table 1: The frequency of abuse according to the type of claim
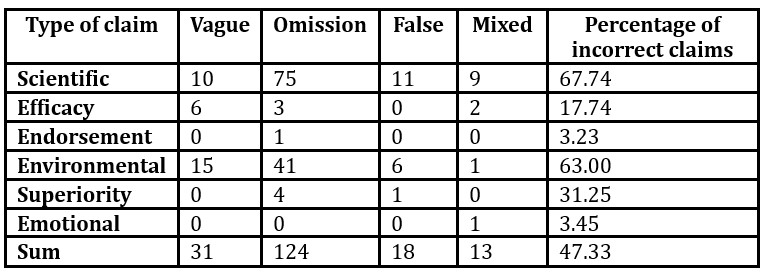
The majority of incorrect claims were omission claims (n=124). These included mainly scientific claims (n=75), primarily those related to the effects of individual ingredients or the ready product and dermatological tests. These claims should be supported by research and evidence, but manufacturers did not indicate such information on packages. This group also included environmental claims (n=41), especially those related to the biodegradable formula and the percentage of naturally derived ingredients as manufacturers did not inform on the methodology on which the content of these ingredients was calculated, e.g., in accordance with the standard 16128-2. In addition, superiority (n=4) and efficacy (n=3) claims were identified in this group.
Far fewer claims were classified as vague (n=31), including environmental (n=15), scientific (n=10) and efficacy (n=6) claims. These were mainly claims difficult to interpret and assess their correctness, e.g. “I’m eco”. Regarding the effects of the individual ingredients and the expected effects, most of the claims were too vague and did not contain any evidence to support the information provided, e.g., “firming gel” without indicating which ingredients are responsible for these properties or on the basis of what studies they were found.
False information was also found among the claims under examination (n=18). This concerned scientific (n=11), environmental (n=6) and superiority (n=1) claims. Most irregularities were related to the declaration of the use of a given ingredient, the presence of which was not confirmed by the composition analysis (n=6) or indicated its replacement with a substitute (n=2), e.g., instead of an organic strawberry extract, there was a wild strawberry flavour. There was also a discrepancy regarding the amount of an ingredient in a cosmetic product (n=1), where the manufacturer declared that 99% of the product was aloe vera juice. However, this juice came second in the composition, and in compliance with Regulation (EC) 1223/2009, the ingredients should be listed in decreasing order, so it is not possible for aloe vera juice to account for 99% of the ingredients. In addition, irregularities also concerned the indication that the gel is a mild product while the analysis of the composition revealed the presence of many substances with an allergenic potential (n=2). False information also referred to the claim that the ingredients present in the formula were natural while the composition analysis did not confirm it. Manufacturers used the terms natural ingredient and an ingredient of natural origin interchangeably, which is a mistake. A superiority claim indicated that the natural ingredients made the product 100% safe, which is also false.
The smallest set of abuses were mixed claims (n=13), which could not be classified in any of the above groups as they included the features of both vague and omission claims. Among them, scientific (n=9), efficacy (n=2), emotional (n=1) and environmental (n=1) claims can be distinguished.
Considering the legal aspects, all of the claims classified as false ones did not meet the criteria set out in Commission Regulation (EU) No 655/2013. Moreover, the analysis revealed the presence of additional 3 non-compliant claims, which were claims of the “free from” type:
- “trustworthy product: contains no synthetic colorants and fragrances”, which may imply that a product with synthetic colorants and fragrances cannot be trustworthy,
- “product contains no colorants, oils or aromatic substances that may cause irritation and allergies’, which may imply that dyes, oils and fragrances are irritating and allergenic,
- “does not contain: SLS, SLES, allergens*, extracts and synthetic colorants, mineral oils (*in accordance with Regulation 1223/2009)”, which fulfills the premise for the abuse of the compliance criterion.
Despite numerous irregularities identified, slightly more than half of the claims placed on the packages of natural cosmetics were correct (n=207) and did not violate the requirements of the law and other documents regulating the issues of cosmetic product labelling.
Conclusions
The provision of information on cosmetics is subject to the so-called co-regulatory system, which consists of legal regulations, self-regulations and good practices. These elements create a system that ensures protection of consumers and entrepreneurs. Unfortunately, despite the common requirements and guidelines developed for all cosmetic products, the lack of a precise definition of a natural cosmetic and the lack of detailed regulations in legal provisions, as well as the lack of knowledge about or non-compliance with the criteria for substantiation of claims are conducive to unfair practices by manufacturers, which take the form of false claims about a product.
As shown by the above study, manufacturers willingly place various marketing claims on the packages of natural shower gels. On average, there are about 7 claims per one tested package. The analysis of these claims showed that they most often relate to the intended use of the cosmetic and the content of active ingredients along with a description of their action. Manufacturers also provide the percentage of natural ingredients in the product, but only few choose to be certified by an external body or to provide the methodology with which the content of these ingredients was calculated. It is also common to indicate that a product is suitable for vegans.
Unfortunately, irregularities were found in almost half of the claims identified, and they mainly related to a lack of presentation of evidence to support the claims made. Only a dozen or so claims did not meet the legal requirements and related to the lack of the declared ingredient in the product composition, the declaration of too high an amount of an ingredient than appeared from the product composition and the use of “free from” claims.
This proves that the actions taken by EU bodies and the growing interest of various entities in greenwashing have the intended effect; on the other hand, it shows that there is still a need to specify the issues related to the claims on cosmetic products, taking into account consumers themselves, who – due to the amount of information provided on packages – are often unable to distinguish reliable information from the so-called marketing tricks (Żakowska, 2017), and due to greenwashing practices occurring in the industry and the lack of trust in manufacturers’ claims, often resign from buying natural cosmetic products (Sadiq, Adil and Paul, 2021).
The above analysis is a preliminary assessment. In order to accurately verify the credibility of the claims made on the packages of natural shower gels, including, for example, the content of active substances or the number of ingredients of natural origin, it is necessary to conduct appropriate laboratory tests and make an assessment based on the manufacturer’s reports and test protocols.
Acknowledgment
The publication was financed from a subsidy granted to Cracow University of Economics.
References
- Carlson, L. (1993) ‘A Content Analysis of Environmental Advertising Claims: A Matrix Method Approach’, Journal of Advertising, 22 (3), 27-39.
- Chandon, L. (2020) ‘Do Claims about the Naturalness and Dose of Cosmetics Ingredients Affect the Public’s Perception of Their Safety?’, J, 3 (3), 299-312.
- Commission Regulation (EU) No 655/2013 of 10 July 2013 laying down common criteria for the justification of claims used in relation to cosmetic products. OJ L 190/31.
- Cousté, N.L., Martos-Partal, M. and Martínez-Ros, E. (2012) ‘The Power of a Package. Product Claims Drive Purchase Decisions’, Journal of Advertising Research, 52 (3), 364-365.
- Fowler, J.G., Reisenwitz, T.H. and Carlson, L. (2015) ‘Deception in cosmetics advertising: Examining cosmetics advertising claims in fashion magazine ads’, Journal of GlobalFashion Marketing, 6 (3), 194-206.
- Gajewska, E. (2020), Przejawy mody językowej w prasowych reklamach kosmetyków przeznaczonych dla kobiet, Język. Tożsamość. Wychowanie III, Feruga, K. and Ostrowska-Knapik, A. (ed), Wydawnictwo Naukowe Akademii Techniczno-Humanistycznej, Bielsko-Biała.
- Hsu, C.L., Chang, C.Y. and Yansritakul, C. (2017) ‘Exploring purchase intention of green skincare products using the theory of planned behavior: Testing the moderating effects of country of origin and price sensitivity’, Journal of Retailing and Consumer Services, 34, 145-152.
- ISO 16128-1:2016. Guidelines on technical definitions and criteria for natural and organic cosmetic ingredients and products — Part 1: Definitions for ingredients.
- ISO 16128-2:2017. Cosmetics — Guidelines on technical definitions and criteria for natural and organic cosmetic ingredients — Part 2: Criteria for ingredients and products.
- Mintel, (2020). Global Beauty and Personal Care Trends 2030. [Online]. [Retrived 10, 2022]. Available: https://www.mintel.com/beauty-trends-2030.
- Naturativ, (2018). Raport Slow Life w Polsce 2018. [Online]. [Retrived March 5, 2022]. https://www.naturativ.pl/raportslow/.
- Nielsen, (2020). Nielsen: Pielęgnacja i higiena skóry — sprzedaż w obu kategoriach rośnie rok do roku. [Online]. [Retrived January 10, 2022]. https://www.wiadomoscikosmetyczne.pl/artykuly/nielsen-pielegnacja-i-higiena-skory-sprzedaz-w-obu,64542/2.
- Organic Trade Association, (2021). Organic industry survey 2021. [Online]. [Retrived February 22, 2022]. https://ota.com/market-analysis/organic-industry-survey/organic-industry-survey.
- Pawlik, A., Niewęgłowska-Wilk, M., Kalicińska, J. and Śpiewak, R. (2017) ‘Kosmetyki „naturalne”, „biologiczne” i „ekologiczne”. Gwarancja bezpieczeństwa czy marketing?’, Kosmetologia Estetyczna, 2, 125-128.
- Regulation (EC) No 1223/2009 of The European Parliament and of The Council of 30 November 2009 on cosmetic products. OJ EU L 342/59.
- Report from The Commission to The European Parliament and The Council on product claims made based on common criteria in the field of cosmetics. COM/2016/0580 final.
- Sadiq, M., Adil, M. and Paul J. (2021) ‘An innovation resistance theory perspective on purchase of eco-friendly cosmetics’, Journal of Retailing and Consumer Services, 59.
- Statista, (2019). Market value for natural and organic beauty worldwide 2018-2027. [Online]. [Retrived March 5, 2022]. https://www.statista.com/statistics/673641/global-market-value-for-natural-cosmetics/.
- Sub-Working Group on Claims, (2017). Technical document on cosmetic claims. [Online], [Retrived March 1, 2022]. https://ec.europa.eu/docsroom/documents/24847.
- Trivedi, M. (2019) ‘Glimpses of green consumerism and steps towards sustainability’, Journal of Management, 6 (3), 35-41.
- Wiadomości kosmetyczne, (2020). PMR: 5 najważniejszych trendów oddziałujących na rynek artykułów kosmetycznych w 2020 roku. [Online]. [Retrived March 1, 2022]. https://www.wiadomoscikosmetyczne.pl/artykuly/pmr-5-najwazniejszych-trendow-oddzialujacych-na-ry,66625/3.
- Żakowska, H. (2017) Opakowania a środowisko. Wymagania, standardy, projektowanie, znakowanie, Wydawnictwo PWN, Warsaw.
- Żyngiel, W. and Platta, A. (2014) ‘Oczekiwania konsumentów wobec preparatów kosmetycznych pochodzenia naturalnego wykorzystywanych w zabiegach SPA &Wellness’, Handel Wewnętrzny, 1 (354), 324-333.




